English
 2025/3/24 8:14:57
2025/3/24 8:14:57
 758
758
- I. Background and Organizational Structure
According to the conclusions of the 2023 Executive Yuan Biotechnology Industry Strategy Advisory Council (BTC), three major strategies were proposed to promote the development of the smart healthcare industry:
- Industry Acceleration: The development of smart healthcare should address aspects such as educational systems, matchmaking mechanisms, certification processes, experience exchange between academia and industry, the formation of cross-disciplinary national teams, and integration into national and commercial insurance. The focus is on developing AI services and human-centered innovative products based on medical big data, with an emphasis on cross-hospital and international validation.
- Infrastructure Innovation: Advancements in the smart healthcare industry rely on the integration of HIS and FHIR systems within the healthcare system, requiring inter-ministerial collaboration. The government has initiated plans for next-generation medical information systems and should ensure these plans adhere to the core principles of "next-generation" and "Public-Private Partnership (PPP)".
- Data Governance: Current and future technology initiatives must establish mechanisms for data interoperability and usability to ensure data availability and compatibility.
To realize the BTC's vision, the Ministry of Health and Welfare's Information Management Office plans to establish three major AI centers and an AI SMART Marketplace to foster the smart healthcare ecosystem. A subsidy plan titled “Establishment of Three Major AI Centers for Next-Generation Digital Health Platforms” has been developed, encouraging hospitals to build cross-hospital data interoperability mechanisms in line with platform specifications. This aims to accelerate AI tool development, promote the medical information industry, improve the information environment, and enhance global competitiveness.
For clinical AI models to be used in major hospitals across Taiwan, TFDA certification is required. This process depends heavily on ensuring model accuracy on external datasets, which necessitates cross-system and multi-tiered patient data for external validation. Hence, the Clinical AI Certification and Validation Center has been established to support AI tools and ensure their adaptability and consistency across systems.
The team structure of this Center is as follows:
- Center Members
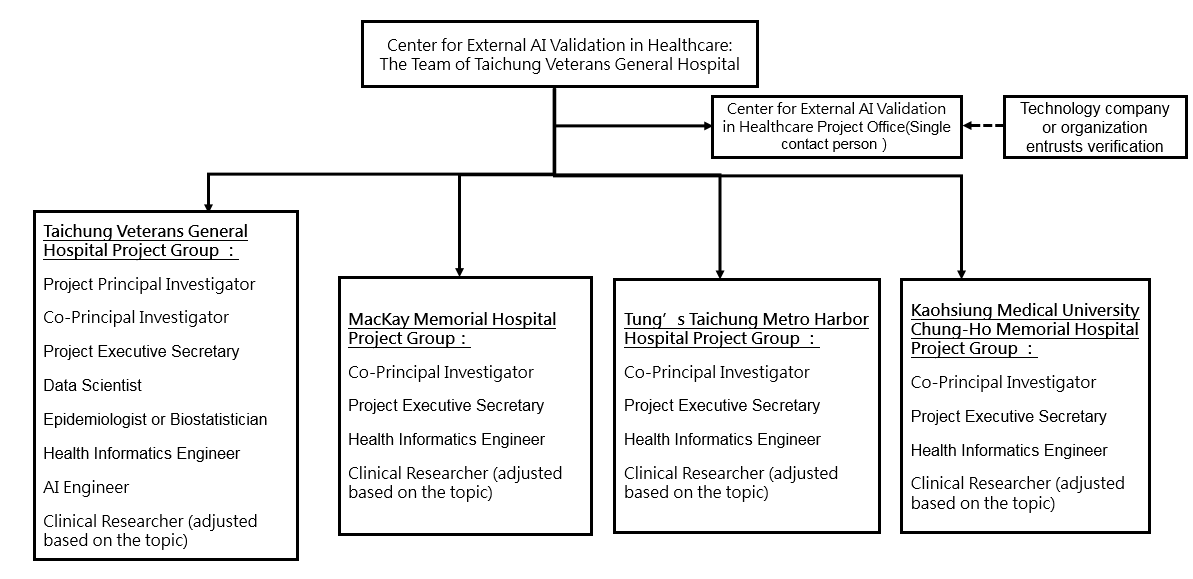
- Team Member Roles and Responsibilities
- Project Office(Single contact person):Acts as the external liaison with the Ministry of Health and Welfare, communicates with the verification vendors or organizations, and coordinates with partner hospitals to implement the project.
- Taichung Veterans General Hospital Project Leader : The principal leader of the project.
- Partner Hospital Co-Project Leader : The hospital's president or vice president, the overall responsible person for the hospital's project implementation.
- Partner Hospital Project Executive Secretary: Responsible for coordinating internal administrative tasks for the hospital's project and acting as the liaison with the project office.
- Health Informatics Engineer: Responsible for extracting required feature data from the EHIS, data cleaning, structuring federated learning, and mapping FIHR codes.
- Clinical Researcher: Responsible for verifying the accuracy of extracted and cleaned data, data labeling, and other related tasks, depending on the research topic.
- AI Engineer: Reviews the AI models from the entrusted organizations, implements federated learning, and verifies the product model packaging.
- Data Scientist: Responsible for big data cleaning, de-identification, automated data cleaning, data security management, and related tasks.
- Epidemiologist or Statistician: Assists in the clinical design and statistical analysis of the verification plan results.
Contact Information
Clinical AI Certification and Validation Center Office
Contact Person: Hsin-Yi Huang, Research Assistant, Department of Critical Care Medicine, Taichung Veterans General Hospital
Phone: (04)23592525 ext. 3977
Address: No. 1650, Sec. 4, Taiwan Blvd., Xitun Dist., Taichung City, Taiwan
Email: jen@vghtc.gov.tw
II. Services Offered:
- Clinical Trial Certification Consultation
This center provides consultation for AI clinical trial certification, aimed at assisting medical institutions, research organizations, and innovative enterprises in swiftly understanding the AI technology certification process for software as a medical device (SaMD). We support clinical trial design, data collection for validation, and reporting of actual validation results.
Our core advantage lies in our integration with multiple medical centers and regional hospitals across northern, central, and southern Taiwan, offering cross-hospital certification experience. We can quickly tailor clinical trial solutions based on the characteristics of clients' AI models, shortening time-to-market, reducing R&D risks, and accelerating smart healthcare development.
Download application forms and self-evaluation forms: Two files will be provided.
- Cross-Hospital Clinical Trial Hospital Matchmaking
Our expert team understands the strengths, patient demographics, and infrastructure of various hospitals, enabling quick identification of suitable clinical trial partners. Based on the AI module’s application area (e.g., image analysis, predictive modeling, diagnostic support), we recommend institutions with sufficient data resources and trial capabilities. We also assist with cross-hospital collaboration challenges such as regulatory compliance, data sharing agreements, IRB applications, and resource allocation to ensure trials proceed smoothly and legally, accelerating cross-hospital validation.
- SaMD Quality Management System (QMS) Consultation
To commercialize AI medical modules, establishing a QMS that meets international standards is essential. Building a QMS for AI-based SaMDs requires collaboration between software developers and manufacturers. We can connect clients with experienced manufacturers familiar with Taiwan’s medical device QMS regulations, supporting the collaborative creation of QMS systems and ensuring compliance with certification requirements—thereby speeding up QMS establishment.
- Medical Device Clinical Trial Design & Clinical Investigation Plan (CIP) Writing
Our experienced team customizes clinical trial plans according to client needs. We analyze AI model features and application scenarios to design scientifically sound and regulatory-compliant trials, including patient population selection, endpoint design, randomization, control group setup, and data collection and analysis strategies.
We also assist in drafting high-quality Clinical Investigation Plans (CIP) covering study background, objectives, methodology, data management, and statistical plans, all compliant with international standards to obtain IRB and regulatory approval, reducing preparation time and regulatory barriers.
- IRB Review Support
IRB approval is essential before launching a clinical trial to ensure ethical design and protect participant rights. Our experienced team offers comprehensive support including document preparation, protocol optimization, and multi-party coordination. We help prepare necessary documents such as the protocol, informed consent form (ICF), ethics statements, and risk assessments. To save time, a lead site will handle the drafting while partner hospitals submit simultaneously. Future efforts will align with plans for joint IRB reviews under the AI Certification and Validation Center.
- SaMD Data Cleaning and Annotation Support
Cross-hospital clinical trials require validated data from multiple institutions. Data cleaning is key to AI model validation as data quality directly affects outcomes. Our center provides standardized recommendations and tool guidance for data cleaning, and trains hospital teams (including physicians, medical staff, and IT engineers) to clean and annotate data according to trial protocols—supporting subsequent centralized analysis or federated learning validation.
- Cross-Hospital Clinical Trials Using Federated Learning
Federated learning allows AI model training across hospitals without moving data off local servers, addressing privacy and compliance concerns—ideal for cross-hospital trials. Partner hospitals offer both centralized integration and federated learning platforms. Our services include collaboration structure design, distributed data workflow setup, federated learning platform deployment and optimization, and real-time model performance and data consistency monitoring.
With our support, clients can validate AI models across multiple sites quickly and securely, reducing trial duration and privacy risks, accelerating real-world AI deployment.
- Clinical Study Report (CSR) Writing
We assist developers and clinical research teams in producing Clinical Study Reports (CSR) that meet international regulatory and academic standards—paving the way for device certification and market entry.
The CSR comprehensively documents trial design, execution, data analysis, and conclusions, providing transparent, detailed information for regulators. Our service includes organizing data analysis results, describing statistical methods, evaluating endpoint achievements, and outlining risks and limitations. We help clients submit high-quality CSRs that comply with regulations, expediting the AI model’s clinical application.



